Are frequent COPD flare-ups keeping you down?
See if the AIRFLOW-3 Clinical Trial is right for you
Over 16 million Americans suffer from chronic obstructive pulmonary disease (COPD), which includes emphysema and chronic bronchitis. COPD makes it hard to breathe, and can also cause wheezing, coughing, and chest tightness. When COPD flares up, it can be quite scary, causing breathing to become extremely difficult. While medications can help with COPD, they do not always effectively control these flare-ups.
If you have COPD and still experience flare-ups despite your medications, you may qualify for the AIRFLOW-3 Clinical Trial. The trial is evaluating Targeted Lung Denervation, a new investigational treatment which may potentially reduce the risk of COPD flare-ups through a one-time, non-surgical, outpatient procedure.
What is Targeted Lung Denervation (TLD)?
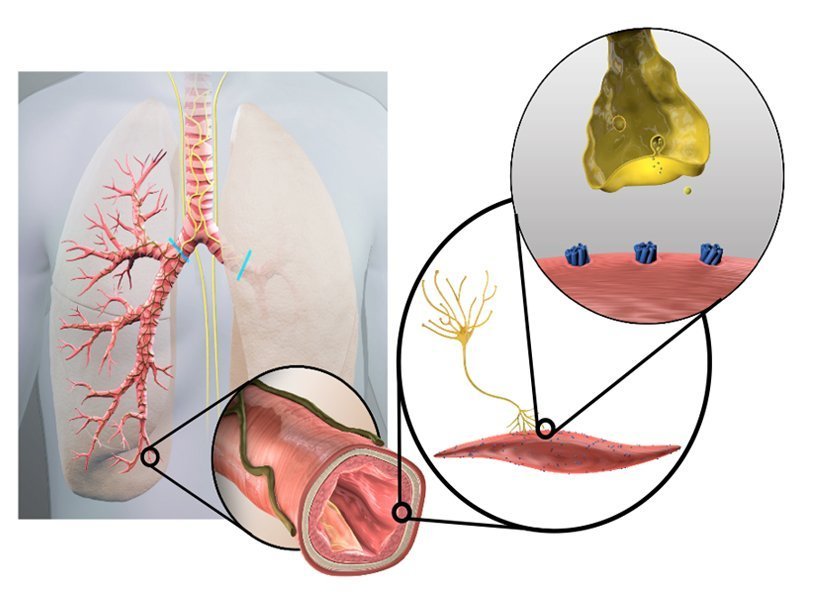
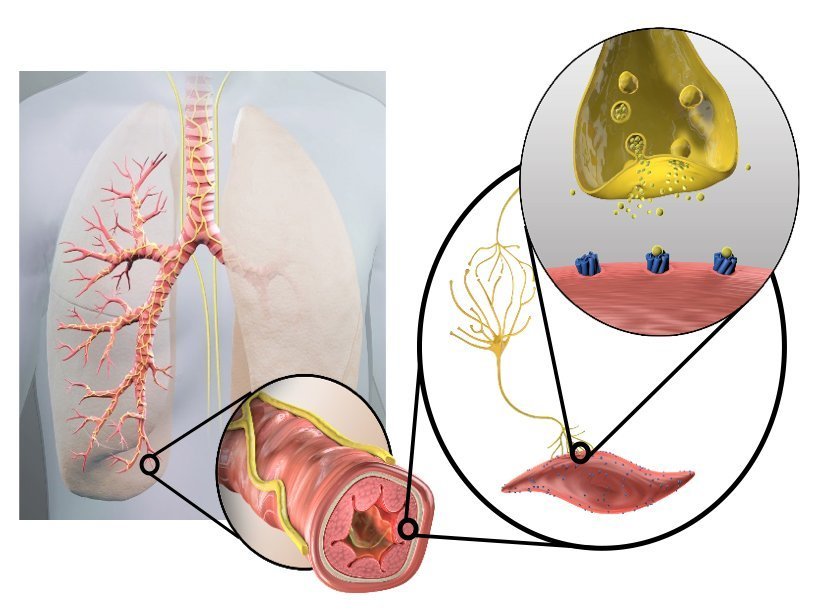
People with COPD have overactive nerves in their airways, which contribute to symptoms and flare-ups that can be mild, moderate or severe. COPD flare-ups can further damage your lungs, making it difficult to recover to your ‘normal’ symptom levels. While medications can help with COPD, they do not always control these flare-ups.
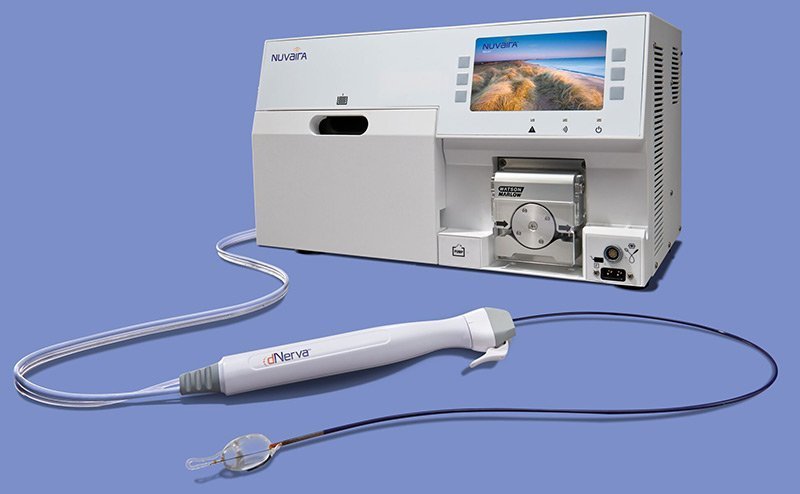
The Nuvaira Lung Denervation System is a new investigational device used in a non-surgical procedure called Targeted Lung Denervation, or TLD. The procedure is designed to reduce airway nerve activity, which may potentially reduce the risk of COPD flare-ups.
The TLD procedure is performed through a standard bronchoscope – a thin flexible tube that is inserted through the mouth into the lungs. Once in place, the Nuvaira System delivers targeted radiofrequency (RF) energy to the nerves located on the outside of the airways. The procedure takes about one hour with most patients returning home the same day.
The Nuvaira System and TLD procedure is currently being offered at select US study centers through the AIRFLOW-3 Clinical Trial.
Why Participate?
If you’re living with COPD and still experience flare-ups despite your medications, you may qualify for the AIRFLOW-3 Clinical Trial. The trial is evaluating Targeted Lung Denervation (TLD), an investigational treatment which may potentially reduce the risk of COPD flare-ups through a one-time, non-surgical, outpatient procedure.
If you qualify and choose to join the study, you will receive:
- Evaluations of your COPD from a local specialist
- The TLD procedure and all study-related visits and tests at no cost
- Ongoing evaluations of your symptoms and results over a 5-year period
- Reimbursement for travel costs to attend study visits
COPD Before TLD